Salinization is an accumulation of salts in the soil and on the surface of the soil. Salts enter the soil through irrigation; they originate either from geological layers, from groundwater or from man-made influences, such as fertilizers.
Crops vary in their reaction to soil salinity. Due to reduced water up-take in relation to the soil salinity, yields will decrease as the plants wilt and eventually die. Crops which are relatively salt-tolerant include date palm, cotton, barley and sugar beet.
Worldwide, approx. 30 million ha of the irrigated total 237 million ha are heavily affected by salinity . In light of the increasing food demand there is an urgent need to stop the progressive salinization of agricultural land. Soil salinization can be combated by drainage, leaching, improved fertilizer management, appropriate agronomic practises, and cultivation of salt-tolerant crop varieties.
Salts
Salts dissolved in water separate into ions (see Table 1), with each affecting soils and crops differently.
Table 1: Main ions of irrigation water
High sodium (Na) content causes the dispersion of the soil aggregates and the deterioration of the soil structure. This reduces the permeability of the soil surface for the infiltration of rainfall and irrigation water and for air exchange: adverse growing conditions for plants are the result.
Chloride (Cl) has no adverse effects on soils but can be toxic to plants if accumulated in the leaves. Fruit trees are sensitive to high concentrations of chloride in irrigation water. When sprinkler irrigation is applied, there is a hazard of leave burn at high Cl-concentrations.
Sulfate (SO4) is essential to plant growth, but at high concentrations in irrigation water it can damage canals and irrigation pipes.
Nitrate (NO3) is a plant nutrient and is often found in groundwater which receives nitrate from percolating irrigation water containing fertilizer residues. Also, effluents from wastewater treatment plants can contain nitrate and end up in groundwater or other receiving waters.
Potassium (K) is a plant nutrient and is often present in percolating irrigation water and effluents.
Magnesium (Mg) is also a plant nutrient. In high concentrations, however, it may inhibit plant growth and reduce crop yields.
Carbonate (CO3) in irrigation water can precipitate Ca++ and Mg++ as carbonates increaseing the adverse effect of sodium.
Measuring salinity
The salinity of irrigation water is given in percentage (%), gram per litre (g/l), milligram per litre (mg/l), parts per million (ppm), total dissolved solids (TDS), or in deciSiemens per metre (dS/m) which is the electrical conductivity (EC). The EC is measured with special meters and is an indicator of the dissolved solids in a given water.
For ease of orientation, Table 2 approximately equates the different measuring units of varying salt contents from different water sources. The EC measurement is dependent on the water temperature: the EC rises with increasing water temperature. As EC is normally measured at 250 C, modern meters automatically adjust the measurement to 250 C.
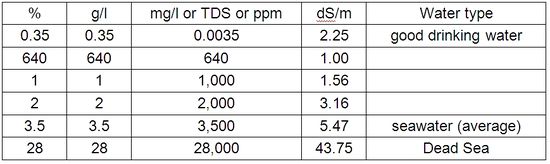
Table 2: Measurements of salt contents of different waters (partly rounded)
The EC measurements give information on the total content of minerals/ions in the water, but do not inform regarding the composition (quality) of the water. Often an index is added to characterize the purpose of the water use, e.g. (ECiw), with ”iw” indicating irrigation water.
Monitoring salinity
It is important to monitor the irrigation water quality with regard to salinity. This is normally done by measuring the electric conductivity of the irrigation water (ECiw). Likewise, it is important to monitor the soil salinity. Soil samples are taken and the (soil) water of a saturated soil paste sample is extracted in labs in order to determine the electric conductivity of the soil water extract (ECe). In the case of constantly applying irrigation water of the same salinity and leaching the soil with a leaching fraction of 15-20%, a steady state will develop in the soil. According to the FAO, the EC of the saturated soil paste extract will, in the long run, be 1.5 times the EC of the irrigation water: ECe = 1.5 ECiw. The GTZ Brackish Water Project in the Jordan Valley also had similar results.[1]
Classification of irrigation water
A rough classification of irrigation water is given in Table 3
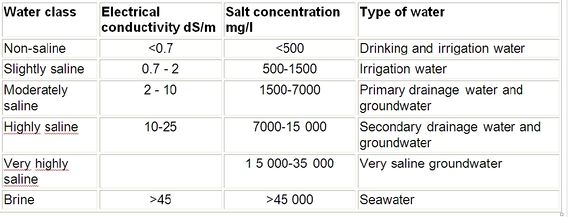
Table 3: Classification of saline waters[2]
Examples of crop tolerance to water salinity in Jordan
Crops react to salinity with reduced yields and eventual death. Examples of highly tolerant, moderately tolerant and sensitive crops are listed in FAO (1985).
[3]The GTZ Brackish Water Project (BWP) monitored economically important crops on farmers’ fields in the Jordan Valley in Jordan. Results indicated that parsley and rucola (rocket) are remarkably tolerant to irrigation water salinity while potato is relatively sensitive (see Figure 1).
Figure 1: Threshold values regarding irrigation water salinity in the Jordan Valley[1]
Agronomic practices to cope with salinity
The application of manure helps to maintain soil fertility and to create good leaching conditions (see following section). The salt index of the fertilizer should be taken into consideration when fertilizer is applied. The salinity index provides a rough estimation of a fertilizer’s possible contribution to soil salinity.
Sometimes saline/brackish water is blended with fresh water to reduce the salinity of irrigation water. However, the Brackish Water Project in Jordan does not recommend this practise as it is not economically viable. Instead, alternate irrigation is suggested. During sensitive growing stages, such as germination, irrigation with freshwater is recommended. Later, when crops are more tolerant, moderately saline water can be applied.
Drip irrigation equipment allows control of salinity through appropriate application of irrigation
water quantities, appropriate timing and high application uniformity. When the dripper lines are covered with mulch, e.g. plastic mulch, less water is needed and thus less salt enters the soil.
Leaching
Leaching is applying additional water to the crop water requirement which helps to wash out salts below the root zone. The excess water should be extracted through subsurface drainage.
Drainage
Drainage releases excess water from irrigated or non-irrigated lands to lower the water table, and collect and dispose of excess water. Salt can run-off through the drainage with the excess water. Large drainage systems with saline excess water can have a significant impact on the salt content downstream.
References
- ↑ 1.0 1.1 GIZ (2003): Guidelines for Brackish Water Irrigation in the Jordan Valley.
- ↑ FAO (1992): The use of saline waters for crop production - FAO irrigation and drainage paper 48. Rome: FAO. http://www.fao.org/docrep/T0667E/T0667E00.htm [2013-02-20].fckLR
- ↑ FAO (1985): Irrigation Water Management: Training Manual No. 1 - Introduction to Irrigation. Chapter 7: Salty Soils. http://www.fao.org/docrep/R4082E/r4082e08.htm [2013-02-20].
FAO (2002): Agricultural drainage water management in arid and semi-arid areas. FAO irrigation and drainage paper 61. Rome: FAO. Annex 1. Crop salt tolerance data. http://www.fao.org/DOCREP/005/Y4263E/y4263e0e.htm [2013-02-20].
Additional information
Akzente/ Nüsse, Andrea (2002): Jordanien. Pioniere wider Willen. Der Feind der Landwirtschaft im Jordantal ist das Salz.
GIZ/Petermann, Thomas (1993): Irrigation and the environment. A review of environment issues.
GIZ/Vallentin, A./Abdel-Jabbar, S./Srouji, F. (2003): Guidelines for Brackish Water Irrigation in the Jordan Valley.
Use of mineralized artesian water to organize irrigated crop farming in the Kyzylkum, Uzbekistan